
DEF HEATING AND COOLING EFFECT ON PRODUCT QUALITY AND SHELF LIFE
- White Papers
- BlueDEF
- November 18, 2019
The most predominant technology to meet the EPA’s stringent NOX and particulate matter emission requirements form 2010 is the SCR (selective catalytic reduction) system, SCR employs the use of DEF (diesel exhaust fluid). Thus, the volume of DEF consumed has grown steadily each year along with the distribution system and the number of customers that use and touch DEF.
As this industry grows, we gain more experience, and our best practices for DEF handling evolves. In this white paper we will tackle the most common questions regarding DEF purity; specifically shelf life and the handling of DEF in hot or cold environments.
DEF Quality under Hot Conditions
The story of DEF and SCR systems began in Europe over ten years ago. Since that part of the world was first to embrace that technology, the ISO (International Standard Organization) put together the first standard on DEF quality, testing, and handling, ISO 22241. As this was a new industry, ISO wrote a conservative standard to ensure the efficacy of the DEF and the safety of the public. As part of the standard, ISO included the recommended shelf life guidelines for DEF as a function of temperature as shown in Table 1.
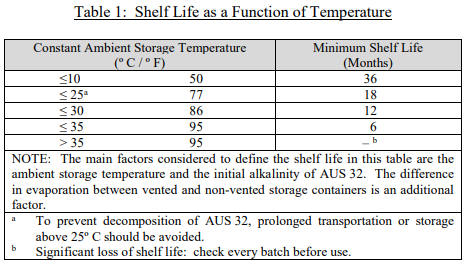

How big of a concern is shelf life temperature and how is the DEF affected?
First, it is important to note that this table shows recommended shelf life at a constant temperature. Thus, for example, if DEF was stored in an area heated to 95ºF in the day, but it cooled to 77ºF at night, the DEF shelf life would be somewhere between six and eighteen months. Moreover, since that length of time includes as least one winter, it is likely there was a Page 3 period where the DEF was much below 77ºF. For this reason, we are more than comfortable stating the shelf life for DEF is a minimum of one year.
What happens in theory that could put the DEF out of specification while sitting in a warehouse from a chemical perspective?
It is not possible for formaldehyde, biuret or any of the metal ions to appear in solution when product sits on a shelf for an extended period of time. The only parameter that could change is alkalinity as ammonia.
With heat and time, some of the urea can react with the water to form ammonia and CO2. Both of those compounds are highly dissolvable in the water itself. The irony is ammonia is the active agent in the SCR system. When DEF is injected into the SCR system, which is at a very high temperature, the first reaction is urea with the water to form ammonia. It is the ammonia that reacts with the NOX over the catalyst to produce N2 and water.
As you can see the “decomposition” of DEF on the shelf does not harm it at all. Yes, possibly some amount of urea or ammonia could be lost to this reaction on the shelf, but the effect on the quality of the DEF is negligible. Even though the ISO standard has this limit on ammonia, being over this particular spec does not affect the performance of the DEF.
So why did the ISO committee include these shelf life guidelines?
Ammonia has a strong chemical smell and there was concern that the consuming public would be adverse to using DEF if it had a chemical smell. With twelve years into Europe using DEF and eight years for the US, shelf life has not been a problem. In addition, the DEF market has reached a consumption level that there is no reason for anyone not to be turning their inventory, even on a store shelf, within a year. Overall, the shelf life for DEF should not pose a concern.
DEF Quality under Cold Conditions
What happens when DEF freezes, either in a container or in equipment, while in use?
To account for this potential issue, the concentration of 32.5% urea in DEF was chosen because it is the eutectic point for urea/water solutions and has the lowest freezing point of any urea/water mixtures, 12º F. The eutectic point is a critical parameter. It is the point at which the mixture will freeze and thaw just like water and ice. When frozen 32.5% urea DEF thaws, it will be entirely liquefied with no residual solid. This is key for safe winter operations of equipment that might experience DEF freezing in tanks.
What happens when the urea concentration exceeds 32.5%?
When the DEF begins to cool, urea will “salt out,” or crystalize out of solution and sink to the bottom of the tank or vessel. As the temperature continues to drop, the urea with continue to salt out until the urea concentration reaches the eutectic point, 32.5%, and then freeze as noted above. If the temperature stays cold long enough, the DEF will freeze solid and become a mixture of pure salted out urea and frozen DEF. Table 2 shows the temperature at which urea/water solutions of varying concentrations will begin salting out.
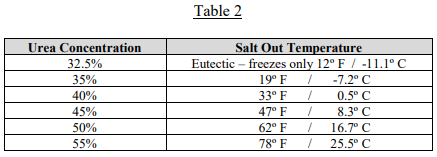

When a frozen urea/water solution thaws, the reverse of the chemistry noted above will occur. As the temperature gets above 12º F, the frozen DEF will thaw, leaving only the salted-out urea. Depending on the concentration of the urea/water solution, as the liquid warms, this salted out urea will redissolve to the concentration allowed in Table 2 as each temperature is reached.
What happens to DEF when it freezes in a sealed container?
When DEF gets below 12º F freezing may occur. When the temperature gets above 12º F the frozen DEF will thaw.
The concentration of urea in the liquid DEF and solid DEF is the same. If DEF has partially frozen in a sealed container or DEF tank, the liquid DEF can still be used until the remaining DEF thaws. This is important for vehicle operation in cold climates. Vehicles with DEF tanks have heaters to thaw the DEF when the engine is started. As the DEF thaws, the liquid DEF can and will be used as it contains the correct urea concentration.
The most important note to point out is that this freeze/thaw cycle of DEF has no effect on the DEF quality no matter how many times it occurs. A gallon of DEF that has been through ten freeze/thaw cycles is of no less quality than DEF that has never frozen once.
If solid or crystalized DEF is encountered in a sealed container or system and is believed to be above 12º F, then it is very likely that the system was not sealed, water has evaporated, the concentration of urea has increased and what is being observed is likely salted out urea.
Why would a customer see frozen DEF or salted out urea in their mobile equipment or storage systems after the winter?
The science facts are that solid DEF cannot occur above 12º F unless the concentration of urea has increased. This can happen through evaporation or sublimation of water over the winter in equipment that is not air tight. It seems highly unlikely that this can happen in a sealed bottle, drum or tote. If solid is observed in a sealed system that is clearly above 12º F, then some sort of manipulation, i.e., putting already over concentrated DEF back into a container, has occurred or the container is not sealed properly.
If evaporation is occurring, let’s examine how easily the urea/water solutions would re-solutionize upon warming. To evaluate this, urea solutions of 35, 40, 45 and 50% were prepared and frozen and then allowed to thaw. Table 3 shows what was observed.
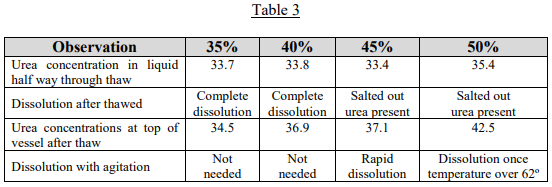

This investigation showed that upon warming, if the concentration of urea is 40% or less, the urea will go back into solution without agitation. Above 40%, when the urea re-dissolves into the liquid, the liquid around the solid urea will become saturated and insulate more urea from going into solution. The concentration of urea in the liquid near the top of the vessel remains below the original concentration.
However, once agitation is provided, which mixes the saturated and undersaturated liquid, the remaining urea goes back into solution quickly in the case of the 45% solution.
The 50% solution takes longer because of another peculiar property of urea/water solutions. When urea is dissolved in water, an endothermic reaction occurs. This is unusual in nature and means that the solution cools as the urea dissolves. When DEF is made at a solutionizing plant, it will cool to 40º when the urea is added to water. When the 50% solution is agitated to redissolve the urea, the solution cools below the 62º F salt out temperature, preventing complete dissolution. However, once the solution warms above 62º F, the urea will go back into solution. It would require considerable water evaporation for a DEF solution to go from 32.5% to 50%; but if a system containing DEF sits all winter completely open to the air, it is possible.
What are some best practices for properly handling DEF and storing DEF over the winter?
- If, after the winter, the users see solid DEF in the DEF tank and the temperature is above 50º, then the urea concentration is probably in the 40% to 45% range. If the temperature is colder, i.e., 35º F, the urea concentration could still be in the 40% to 45% range but might only be in the 35% to 40% range. The concentration could be corrected by adding deionized water to the tank in 10% of the amount of DEF in the tank if at 35º F, or 20% of the volume in the DEF tank if the temperature is above 50º F, and then top up with fresh DEF. This would re-dissolve the salted-out urea, but final urea concentration could be in the 30% to 40% range. Deionized water quality would need to be used. This, of course, assumes the operator has the ability, perhaps from OBD (on board diagnostics), to determine how much fluid is in the DEF tank. It is possible, assuming urea concentration is less than 45% and the DEF tank is less than half full, to just put in fresh DEF. This would dissolve the salted-out urea but would result in a DEF solution in the 35% to 40% range. SCR equipment has on board diagnostics that monitor DEF quality (concentration). The end-user would have to confirm with their equipment provider that having DEF in the 35% to 40% urea concentration range would not affect the OBD.
- Realizing proposition 1 requires the operator to make some judgment calls and assumptions about the quantity and concentration of the DEF, it might be simpler, when crystalized urea is present, and the system is believed to be over 50º F, to clean out the DEF system and begin again with fresh DEF. This would be quite simple. Just fill the DEF tank with tap water and then drain it. The urea will easily dissolve in the water, which is then essentially liquid fertilizer. This can be poured down the drain unless volumes are large in which case the equipment user may wish to check with the local waste treatment facility on proper disposal recommendations. Then refill the system with fresh DEF. The small amount of residual tap water will not harm the SCR system or catalyst.
Best Practices for storing SCR equipment in the Winter
For future winters, SCR equipment users who store their equipment over the winter should drain the DEF tank. The following spring the tank should be filled with fresh DEF. Any frozen DEF or salted out urea will have negligible effect on the fresh DEF.
If SCR equipment has the DEF tank emptied before winter, the DEF must be disposed of properly (check with your state and local waste treatment facility) or put back in an acceptable DEF storage container. The only acceptable DEF storage container would be a drum or tote that had been previously used in DEF service.
Another winter maintenance solution for SCR equipment is to fill the DEF tank to 95% to 100% full. By doing this, the air space will be small, preventing much “breathing room” for the tank and greatly reducing water evaporation. Thus, any water evaporation will have a negligible effect on the urea concentration in the DEF.
In summary, when storing SCR equipment during the winter make sure the DEF tank is either full or empty.
So, this is the science, the effects and the practicality of DEF use in the field as it relates to temperature on DEF. If you have any further questions on the science of DEF and SCR, please call the PEAK Commercial & Industrial DEF technical hotline at 800/477-5847.